what is electrochemistry ?
it is the branch of science which deals with relationship between electrical energy and chemical energy produced during the Redox reaction i.e. how chemical energy produced in the redox reactioncan be converted into electrical energy and vice versa.
it is the branch of science which deals with relationship between electrical energy and chemical energy produced during the Redox reaction i.e. how chemical energy produced in the redox reactioncan be converted into electrical energy and vice versa.
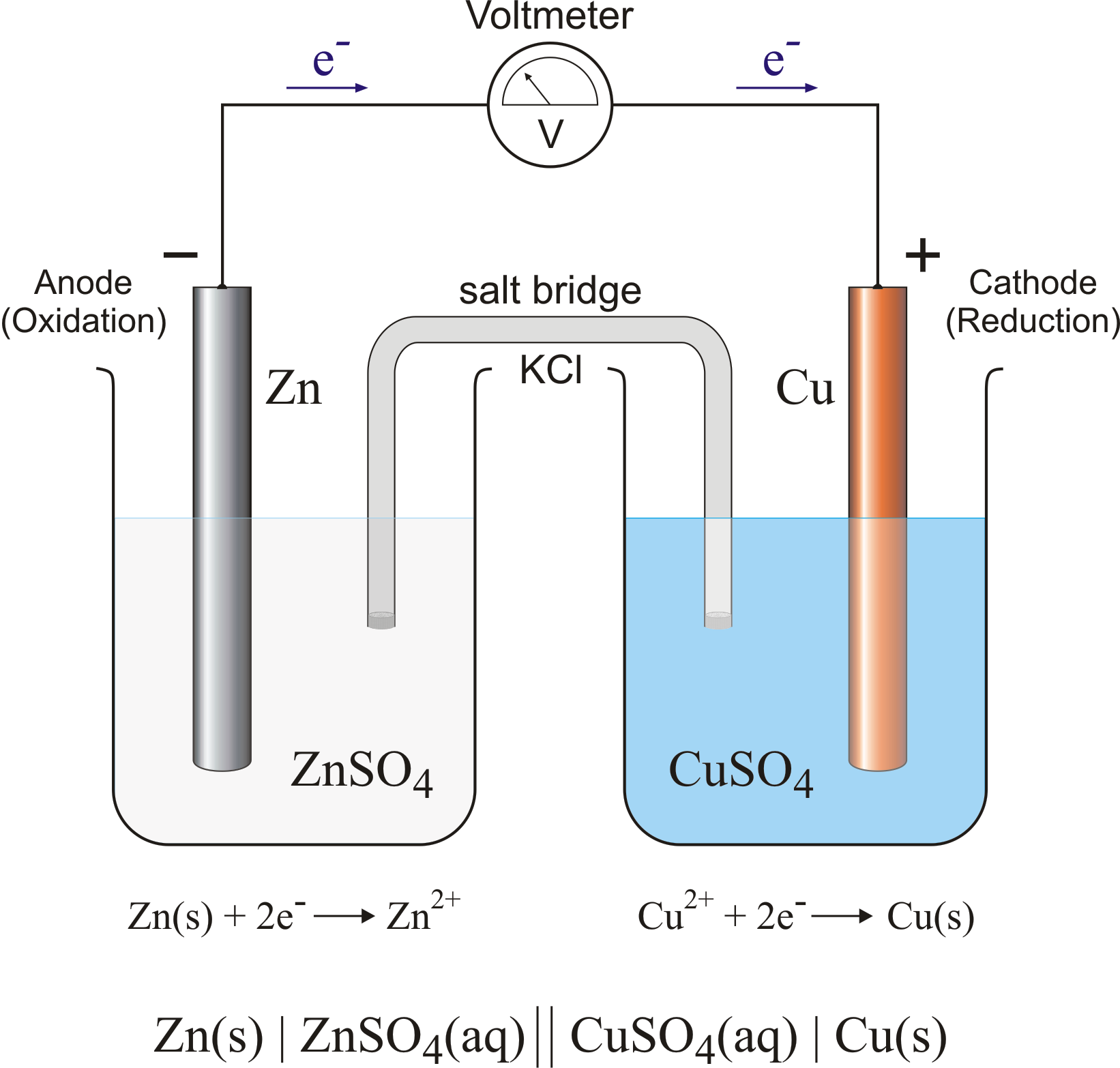
electrochemical cell or Galvanic cell-
A device which convert chemical energy into electrical energy through a Redox reaction Redox reaction consists of two half cell oxidation half and reduction half reaction.
oxidition half cell-
M (s)= Mn+ + ne-
reduction half cell
Mn+ + ne_ = M(s)
galvanic cell usually consists two electrolyte solution into which two electrolyte of different metals are dipped.
Principle
the galvanic cell burns with redox reaction,

*in this cell oxidation and reduction occurs in separate container called half cell
*the electron move from of citation half cell to reduction half cell and current flow from reduuction half cell to oxides and half cell.
⇒ the cell is usually represent as
Zn(s)|Zn2+(aq)||Cu2-|Cu(s)
⇒ The two half reaction are -
At anode-(oxidition take place)
Zn ® Zn2+ + 2 e-
At cathode-(reduction take place)
Cu2+ + 2 e- ® Cu
net reaction is-
Zn +Cu2- ® Zn2+ +Cu
Construction -
Electrochemical series
electrochemical series is arrangement of electrodes in order to increasing electrode potential the standard reduction potential is large number of electrodes has been measured using a standard hydrogen electrodes a reference electrode.
Series
Application of electrochemical series-
1. to predict the relative oxide the oxidising and reducing power-
greater the reduction potential more easily the substance is reduced and hence is a stronger oxidising agent
example- oxidation power of halogens are in order fluorine> chlorine >bromine >Iodine >astatine
2. to predict weather and metal which reacts with acid to give H2 gas--
only does metal will have a test which have negative value of reduction potential
3. to calculate the standard EMF of the cell-
the EMF of the cell which is the difference between reduction potential ofcathode and anode is determined by the given formula
EMF of cell =(EMF of cathode - EMF of anode)
4. compare relative activity of metals-
greater the oxidation potential of a metal greater is the activity as the result are more active metal can displace list active metal from its solution
Standard electrode potential or hydrogen electrode potential
when a metal is in contact with 1 molar solution of its own ion at 250C it may either undergo oxidation or reduction because of this positive or negative charge is developed on the metal which attract positive charge in the solution hence
" standard electrode potential either tendency of electrode to lose or gain electrons when it is in contact with solution of its own Iron for the is the standard conditioner used that is temperature to 98 K concentration molar and pressure 1 atmospheric pressure the electrode potential is called Standard potential normal hydrogen electrode is used as a reference electrode so it is also known as standard hydrogen electrode potential "
SHE(standard hydrogen potential) - it consists of Platinum foile for wire coated with platinum black dipped into 1 molar solution of hydrogen ion hydrogen gas at 1 ATM pressure is continuously passed through it at 298 K it is a reversible electrode
H2 = 2H+ + 2e-
lead storage battery
A storage cell can operate both as a voltic cell or as an electrical cell. it has both type of properties i.e it has ability to work in both ways,to receive electrical energy and also to supply it. when it operates as a voltaic cell it supply electrical energy and as a result it eventually becomes rundown.then it need to be recharged,the cell operates as an electrolytic cell.
lead storage battery is common example of storage cell.
discharging-
when the lead accumulator is used for supplying electrical energy it is said to be discharging of cell.
The lead electrolyte looses the electrons which flow through the wire i.e.
at anode oxidation takes place
Pb(s) + HSO−
4(aq) → PbSO
4(s) + H+(aq) + 2e−
4(aq) → PbSO
4(s) + H+(aq) + 2e−
at cathode reduction takes place
- PbO
2(s) + HSO−
4(aq) + 3H+(aq) + 2e− → PbSO
4(s) + 2H
2O(l)
The total reaction can be written as
- Pb(s) + PbO
2(s) + 2H
2SO
4(aq) → 2PbSO
4(s) + 2H
2O(l)
charging
During the discharging ,pbso4 is ppted at both electrodes. when pbso4 compeletly covers both the electrode (electrode and cathode), the cell stops functioning as a voltaic cell for further use it need to be recharged.
recharging is done by passing an external EMF which is greater than 2v so that the reaction takes place.
reactions are reversed of the discharging of battery
corrosion-
corrosion is the process of gradual degradation of a metal due to the unwanted chemical or electrochemical interaction of metal with its environment.
Ex- rusting of iron, termination of silver
electrochemical theory of corrosion or wet theory of corrosion-
corrosion takes place by the transfer of electrons from anodic part of metal to the cathodic part through the conducting solution is known as electrochemical corrosion
it take place mostly under wet or moist condition through the formation of short circuited Galvanic cell
mechanism of electrochemical theory of corrosion -
1.Electrochemical corrosion involve a separate anodic and cathodic parts between which current flow through the conducting medium
2. The occurrence of oxidation at anodic area which generate metallic Ion
3. Non metallic Ion like ah, two are formed at the cathode area next the diffusion of metallic and nonmetallic iron towards Each Other through conducting medium formation of corrosion produced somewhere between anodic and cathodic areas
Reaction at anode
Fe(s) → Fe2+(aq) + 2e- Equation 1
Reaction at cathode
O2(g) + 2H2O(l) + 4e- → 4OH-(aq) Equation 2
Overall
2Fe(s) + O2(g) + 2H2O(1) → 2Fe2+(aq) + 4OH-(aq) Equation 3
Prevention of corrosion-
(i) by painting the surface.
(ii) by coating the surface with a thin .film of oil or grease
(iii) by electroplating iron with some non-corrosive metal such as nickel, chromium, copper, etc.
(iv) Lamination with plastics
(v) by coating
lubricant
A substance (such as grease) capable of reducing friction, heat, and wear when introduced as a film between solid surfaces to improve the efficiency and reducing wear.
ex- oil,grease,graphite.
lubrication
lubrication may be define as " the reduction of friction and wear between the surface. this type of substances are called lubricant & this process is called lubrication.
classification of lubricant
| ||
Solid Lubricants : | ||
| A solid lubricant is basically any solid material which can be placed between two bearing surfaces and which will shear more easily under a given load than the bearing materials themselves. The coefficient of friction in dry lubrication is related to the shearing force and the bearing load. Two primary property requirements are : 1. Material must be able to support applied load without significant distortion, deformation or loss in strength. 2. Coefficient of friction and the rate of wear must be acceptably low. | ||
Advantages & disadvantages of solid lubricants are listed in Table- | ||
| Advantages and Disadvantages of solid lubricants. | ||
| Semi-Solid Lubricant : Grease |
| In layman’s language Grease is: A black or yellow sticky mass used in the bearings for lubrication purpose. Lubricating greases consist of lubricating oils, often of quite low viscosity, which have been thickened by means of finely dispersed solids called thickeners. It consist of base oils(75 to 95%), additives(0 to 5%) and minute thickener fibers(5 to 20%). |
 Semi solid lubricant. |
| Base Oil :- Many different types of base oil may be used in the manufacture of a grease, including petroleum (napthenic: more popular, parafinic) and synthetic (PAO's, esters, silicones, glycols). The viscosity of the base oil is the most significant property. A lighter, lower viscosity base oil is used to formulate low temperature greases, while heavier, higher viscosity base oil is used to formulate high temperature greases. Additives :- Chemical additives are added to grease in order to enhance their performance. Performance requirements, compatibility, environmental considerations, color and cost all factor into additive selection. Solid lubricants such as graphite, MoS2, EP additives are few examples. Thickener :- The two basic types of thickeners are organic thickeners and inorganic thickeners. Organic thickeners can be either soap-based or non-soap based, while inorganic thickeners are non-soap based. Simple soaps are formed with the combination of a fatty acid or ester (of either animal or vegetable origin) with an alkali earth metal, reacted with the application of heat, pressure or agitation through a process known as saponification. The fiber structure(Fig. 4.48) provided by the metal soap determined the mechanical stability and physical properties of the finished grease. In order to take on enhanced performance characteristics, including higher dropping points, a complex agent is added to the soap thickener to convert it to a soap salt complex thickener. The greases are then referred to as "complexes“. A classification greases based on simple and complex soap thickeners is listed in Table 4.16. The most commonly economic grease is lime(calcium) base grease (max. temperature 55-800C). Soda(Sodium) base grease (max. temperature 90-1200C) is preferred over lime based grease in rolling bearings. Complex Grease :- Complex grease is similar to a regular grease except that the thickener contains two dissimilar fatty acids, one of which is the complexing agent. This imparts good high temperature characteristics to the final product. |
Synthetic lubricants
- Polyalphaolefins (PAO)
Polyalphaoleins are the most popular synthetic lubticant. PAO’s chemical structure and properties are identical to those of mineral oils.
Polyalphaoleins (synthetic hydrocarbons) are manufactured by polymerization of hydrocarbon molecules (alphaoleins). The process occurs in reaction of ethylene gas in presence of a metallic catalyst.
Polyalphaoleins (synthetic hydrocarbons) are manufactured by polymerization of hydrocarbon molecules (alphaoleins). The process occurs in reaction of ethylene gas in presence of a metallic catalyst.
- Polyglycols (PAG)
Polyglycols are produced by oxidation of ethylene and propylene. The oxides are then polymerized resulting in formation of polyglycol.
Polyglycols are water soluble.
Polyglycols are characterized by very low coefficient of friction. They are also able to withstand high pressures without EP (extreme pressure) additives.
Polyglycols are water soluble.
Polyglycols are characterized by very low coefficient of friction. They are also able to withstand high pressures without EP (extreme pressure) additives.
- Ester oils
Ester oils are produced by reaction of acids and alcohols with water.
Ester oils are characterized by very good high temperature and low temperature resistance.
Ester oils are characterized by very good high temperature and low temperature resistance.
- Silicones
Silicones are a group of inorganic polymers, molecules of which represent a backbone structure built from repeated chemical units (monomers) containing Si=O moieties. Two organic groups are attached to each Si=O moiety: eg. methyl+methyl ( (CH3)2 ), methyl+phenyl ( CH3 + C6H5 ), phenyl+phenyl ( (C6H5)2 ).
Gaseous lubricant
Lubrication with a gas is analogous in many respects to lubrication with a liquid, since the same principles of fluid-film lubrication apply. Although both gases and liquids are viscous fluids, they differ in two important particulars. The viscosity of gases is much lower and the compressibility much greater than for liquids. Film thicknesses and load capacities therefore are much lower with a gas such as air (see Table 1). In equipment that handles gases of various kinds, it is often desirable to lubricate the sliding surfaces with gas in order to simplify the apparatus and reduce contamination to and from the lubricant.
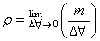


0 Comments