what is fluid mechanics?
Fluid mechanics is a branch of applied mechanics that is concerned with the statics and dynamics of liquids and gases. The analysis of the behavior of fluids is based upon the fundamental laws of applied mechanics that relate to the conservation of mass, energy, and momentum.
OR,
A fluid is a substance that deforms continuously under the application of shear stress.
FLUID PROPERTIES
1. Density- Density is the ratio of the mass of a given amount of the substance to the volume it occupies.
D=m/v
where m=mass
v= volume

( it is the dimensionless term)
⇒note that at 4∘ c water have maximum density.
⇒ we can use other fluid at the place of water if we take other fluid as a reference fluid.
S=13.6 for mercury & S= 1 for water.
Specific volume is denoted by the symbol ‘v’. Its unit is m3/kg.
v=vol./mass
The degree of compressibility of a substance is characterized by the bulk modulus of elasticity, K, defined as
Surface tension is the surface force that develops at the interface between two immiscible liquids or between liquid and gas or at the interface between a liquid and a solid surface.
In short, it is apparent tensile stresses which act at the interface of two immiscible fluids.
Because of surface tension, small water droplets, gas bubbles, and drops of mercury tend to maintain spherical shapes.
ex.-
water =0.03 atm
Dimensional Formula: [ML-1T-2]
⇒ Newton's law of viscosity-Newton's viscosity law states that the shear stress between adjacent fluid layers is proportional to the velocity gradients between the two layers.
A FALL in a liquid below the level of zero pressure due to a net downward force produced by the attraction of the same molecules of a solid.mercury has higher cohesive forces compared to adhesive forces.
OR,
A fluid is a substance that deforms continuously under the application of shear stress.
CONTINUUM CONCEPT
The concept of the continuum is a kind of idealization of the continuous description of matter where the properties of the matter are considered as continuous functions of space variables. Although any matter is composed of several molecules, the concept of continuum assumes a continuous distribution of mass within the matter or system with no empty space, instead of the actual conglomeration of separate molecules.1. Density- Density is the ratio of the mass of a given amount of the substance to the volume it occupies.
D=m/v
where m=mass
v= volume
2. Specific weight(w) - As we express a mass M has a weight W=Mg. The specific weight of the fluid can be defined similarly as its weight per unit volume.
w=ρg=weight/vol.
where p= density of fluid
g=gravitational acceleration
3. Specific Gravity: Specific gravity is the ratio of the specific weight of the given fluid to the specific weight of the standard fluid. It is denoted by the letter ‘S’. It has no unit.
4. Relative density (Specific gravity, S) -
Specific gravity is the ratio of the fluid density (specific weight) to the fluid density (specific weight) of a standard reference fluid. For liquids water is considered as standard fluid.
⇒note that at 4∘ c water have maximum density.
⇒ we can use other fluid at the place of water if we take other fluid as a reference fluid.
S=13.6 for mercury & S= 1 for water.
5. Specific Volume:
Specific volume is the volume of fluid (V) occupied per unit mass (m). It is the reciprocal of density.Specific volume is denoted by the symbol ‘v’. Its unit is m3/kg.
v=vol./mass
6. Compressibility-
Compressibility may be defined as the ratio of change in volume of the fluid to the original volume. It can be mathematically expressed asThe degree of compressibility of a substance is characterized by the bulk modulus of elasticity, K, defined as
K=- δp/(δv/v)
dimension of K= [ML-1T-2]
where δp represents the small increased in pressure applied to the substance that causes a decrease of the volume by δV from its original volume of V.⇒ compressible fluid-
The fluids in which there is a change in volume, density and specific weight occur due to the application of pressure are called compressible fluids.⇒ incompressible fluid-
The fluids in which there is no change in volume, density and specific weight occurs due to change in pressure are known as incompressible fluids.
7. Surface Tension -
Surface tension is the surface force that develops at the interface between two immiscible liquids or between liquid and gas or at the interface between a liquid and a solid surface.
In short, it is apparent tensile stresses which act at the interface of two immiscible fluids.
Because of surface tension, small water droplets, gas bubbles, and drops of mercury tend to maintain spherical shapes.
8.vapor pressure-
The vapor pressure of a liquid is the equilibrium pressure of a vapor above its liquid (or solid); that is, the pressure of the vapor resulting from evaporation of a liquid (or solid) above a sample of the liquid (or solid) in a closed container.ex.-
water =0.03 atm
Dimensional Formula: [ML-1T-2]
Factors That Affect Vapor Pressure
- Surface Area: the surface area of the solid or liquid in contact with the gas has no effect on the vapor pressure.
- Temperature: At a higher temperature, more molecules have enough energy to escape from the liquid or solid. At a lower temperature, fewer molecules have sufficient energy to escape from the liquid or solid.
- Molecule type: if molecule bonding is small then v.p. will less and vice versa.
9. viscosity-
when fluid flow through any medium then the fluid motion is resisted by every layer of fluid to the adjacent layer of fluid.- Cohesion- with like molecules (i.e same fluid's molecules)
- Adhesion-with unlike molecules (i.e foreign material's molecule)
10. capillary rise and fall-
A rise in a liquid above the level of zero pressure due to a net upward force produced by the attraction of the water molecules to a solid surface. water molecules have higher adhesive force compared to its an intermolecular force(cohesive force)A FALL in a liquid below the level of zero pressure due to a net downward force produced by the attraction of the same molecules of a solid.mercury has higher cohesive forces compared to adhesive forces.
- the molecules of mercury are attracted to their own molecules and will not stick with the capillary wall.
- they will attract towards the surface of capillary and thus gradually increase the level of water in capillary compared to the general level of water.







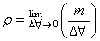


0 Comments